Speed of reaction is a very important topic in Chemistry and there are multiple concepts with which you should know to ace your exams.
Rate of reaction refers to the speed at which a chemical reaction proceeds (takes place) and this speed can vary dramatically.
The speed at which a chemical reaction takes place is mainly dependent upon two factors. Those are:
- Frequency of collisions:
The more often molecules collide with each other, the greater the rate of reaction.
- The energy of collisions:
When the molecules strike each other with greater energy, the reaction proceeds faster. In other words, the forcefully the molecules collide, the greater will be the rate of reaction.
This was all about the introduction of this topic. Now, let's discuss some important concepts about this topic.
How do we measure the speed of a reaction:
To know the rate at which the reactants are utilised or the rate at which the products are formed, we can measure the time taken for the reaction to complete.
Note: If the reaction is faster, it will take less time to complete. However, if the reaction is slower, it will take more time to proceed.
To find the speed at which a reaction takes place, you can:
- Measure the change in mass
- Measure the amount of gas released
Let us design an experiment on how you can measure the change in mass and find the rate (speed) of reaction.
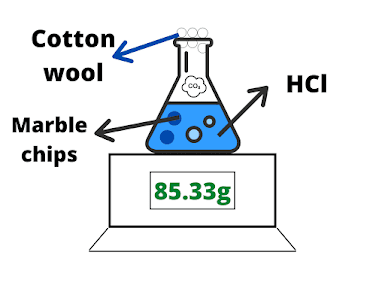
Measuring the change in mass:
Secondly, add hydrochloric acid (HCl) and marble (calcium carbonate).
Place cotton wool at the top of the conical flask to close it so that the gas (carbon dioxide) does not escape. This is the time when you should note the initial mass of the contents.
- Start a stopwatch as soon as the acid and marble start reacting.
Note down the mass at different internals (for example measure the mass after each minute).
- Note the mass of the contents once the reaction is over and stop the stopwatch immediately.
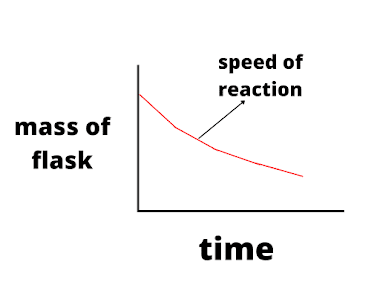
- Plot the graph of the change in mass (on Y-axis) against time (on X-axis). The gradient of the graph will represent the rate (speed) of reaction.
In the reaction mentioned above, the graph would look like:
This was one of the methods to find out the rate of a reaction. Now, let's take a look at another method to find the speed at which a reaction proceeds.
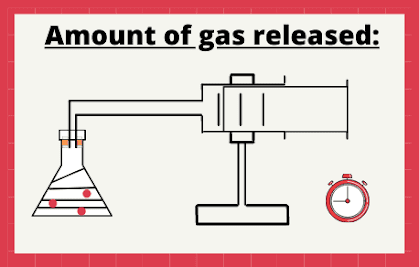
Measuring the amount of gas released:
In this method, you would require a conical flask, gas syringe, stopwatch and a stand.
Firstly, add dilute hydrochloric acid (HCl) to the conical flask containing calcium carbonate (marble).
Note: When an acid reacts with a carbonate, carbon dioxide gas is released.
Secondly, measure the amount of gas released after each minute. Then, plot the graph of the volume of gas produce (on Y-axis) against time (X-axis).
In the graph, the gradient will indicate the speed of the reaction (the larger the gradient, the higher the speed of a reaction).
Initially, the gradient of the graph will increase but as the reaction proceeds, the gradient decreases. There will be a time when the graph will show a horizontal line which would indicate that the gradient is zero.
This is because, after a certain period, no more carbon dioxide will be produced.
These are the two methods which you can use to calculate the speed of reaction. Now, you should also know some of the factors that affect the rate of a reaction.
Factors affecting the rate of a reaction:
Size of the reactant:
The smaller the size of a particle, the higher the rate at which a reaction would proceed. Why is this so?
This is because the particles with smaller size have a larger surface area (the exposed area).
Similarly, larger surface area means more effective collisions and therefore, the speed would also increase.
However, if the size of a particle is bigger, the rate (speed) of reaction would be lesser because the surface area would also be less. This will be because the collisions would also decrease.
Further reading:
In order to put this information into an experiment, add some marble to dilute hydrochloric acid and calculate the amount (volume) of carbon dioxide produced.
Note that everything in the experiment (such as temperature and amount of acid) should be kept constant. The size of the marble should only be varied.
You will conclude that when the size of a marble is smaller, the amount of carbon dioxide produced will be greater. Now, what do you think would be the effect of pressure? Let me tell.
The pressure:
When the pressure is increased, the speed of reaction would also increase because there will be more effective collisions.
When the particles will collide more frequently, the reaction would proceed faster. You can increase the pressure by decreasing the volume because the particles will get closer to each other.
But, if you decrease the pressure, the speed would decrease because there will be less effective collisions.
When the particles will collide less frequently, the reaction would proceed slower. It is to note that when you decrease the pressure, the concentration of the gas also decreases.
The concentration of the reactant:
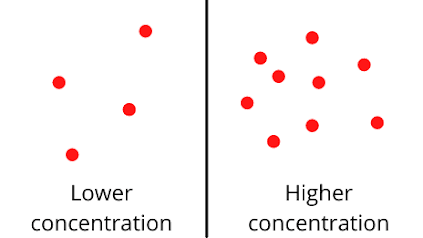
Increasing the concentration would increase the rate of reaction because the particles (reactants) will collide more frequently (effective collision).
This is because higher concentration refers to an increase in the number of particles. As a result, more particles would collide and the reaction would occur rapidly.
If the concentration is less, the reaction rate will decrease because there will be lesser collisions between the particles. The diagram below illustrates what is meant by higher and lower concentration.
If you increase the temperature, the reaction speed would also increase.
Increasing the temperature raises the average kinetic energy (related to motion) of the reactant particles. As a result, the particles move at a faster rate and collide more frequently.
These effective collisions cause the reaction to proceed faster.
However, decreasing the temperature also decreases the rate of reaction because the number of collisions reduces when the average kinetic energy reduces.
In short, temperature and reaction rate are directly related (when one increases, the other also increases and vice versa).
The use of catalysts:
A catalyst is a substance that increases the rate at which a reaction proceeds but remains unchanged at the end of a reaction.
In other words, a catalyst remains unconsumed during a reaction. Enzymes are the catalysts made up from protein and they speed up biochemical reactions.
How do catalysts increase the speed of a reaction?
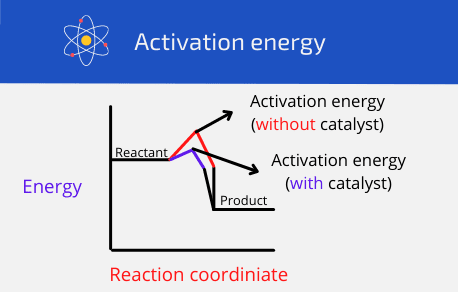
Catalysts lower the activation energy (the energy required for a reaction to proceed) for a reaction. You can also say that a catalyst changed the reaction mechanism.
Moreover, lowering the activation energy means that bonds are broken down more rapidly and therefore, effective collisions also occur. You should know that catalysts only speed up a reaction but they do not affect the amount (volume) in a reaction.
For example, we use Iron as a catalyst in the Haber's process (production of ammonia) and Vanadium pentaoxide (V2O5) as a catalyst in the contact process (for the production of sulphuric acid).
These are some of the factors that affect the rate of a reaction.
Conclusion:
With this, this article about the speed of reaction has come to an end. I hope that all your questions have been answered. Practice plenty of past paper questions to ace this topic.
Some of the topics that are covered are listed below for your guidance.
- How to calculate the speed of a reaction?
- The affect of temperature, pressure, concentration and catalyst on the rate of reaction.
- How does the particle size influence the rate (speed) of a reaction?
Thank you very much for reading and staying with me till the end.
Comments
Post a Comment