Ionic bonding is an important topic in IGCSE Chemistry. Therefore, you should be familiar with some of its important concepts.
So if you want to know about cations, anions and ionic bonds, stay tuned. Now, let's start the topic without further introductions.
What is an Ion?
An ion is a particle with an electric charge (either positive or negative). If a particle gains electrons (like non-metals), then it will have a negative charge.
On the other hand, if a particle loses electrons (like metals), it will have a positive charge. Now moving on to the question I asked you earlier about cations and anions.
Remember that cations are positively charged ions (which means that they lose electrons). Apart, the anions are negatively charged ions (which means that they gain electrons).
Note: The electronic structure of an ion will be similar to that of a noble gas (for example neon, argon and helium).
Now, let's dig a little bit deeper into the topic to discover new concepts as well.
Ionic Bonding Notes:
Metals: When we are talking about metals (such as calcium and potassium), remember that they will lose electrons to achieve an electronic structure that of a noble gas.
What do I mean by that?
Let me explain.
Noble gases are gases that have either two or eight electrons in their outermost shell. This means that they are stable. Thus, they do not lose or gain electrons to become stable.
When it comes to metals, they lose electrons to become stable. Or in other words, they achieve the same state as noble gases have (stable or unreactive).
This is the reason why I referred to their structure as similar to noble gases. I hope this makes sense to you as well now.
Non-metals: They always gain electrons to achieve the electronic configuration of that of a noble gas (become stable). This is the reason why they are negatively charged (because they gain electrons).
Note: The force of attraction between the positive and negative charges (that hold them together) is known as the electrostatic force.
Now, let me tell you about how ionic bonds are formed and how can we deduce their formula?
How ionic bonds are formed?
Let's take an example to understand how the formation of ionic bonds take place.
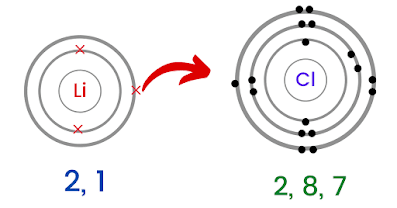
Lithium Chloride (LiCl)
Lithium is an alkali metal (in group 1 of the periodic table). Its electronic configuration is 2, 1. This means that it has to lose one electron to become stable.
Moving on to chlorine, its electronic configuration is 2, 8, 7. This means that chlorine needs only one electron to become stable.
So what happens is that both of these elements form an ionic bond (lithium loses an only electron and chlorine gains one electron) to become stable.
Note: An ionic bond is formed between a metal and a non-metal. For example, sodium chloride (NaCl) is formed between a metal (sodium) and a non-metal (chlorine).
More Resources:
The atmosphere and environment | O Level Notes (5070)
To deduce the formula of an ionic compound, follow the steps mentioned below.
Find out the ions in the compound (in this case lithium and chlorine ion)
Then, write down the ions (for example Li+ and Cl-)
Finally, cross multiply their powers (value on the ions) to find out the formula.
Now moving on, let's talk about the properties of ionic compounds.
Properties of Ionic Compounds:
- They have high melting and boiling point.
The ionic compounds have a high melting and boiling point because the electrostatic forces between the positive and negative charges and very difficult to overcome.
Therefore, high heat is required to break the strong bonds. As a result, they have a high melting and boiling point.
- They conduct electricity in the molten and aqueous state:
In solid-state, they do not have any free moving (mobile) ions or electrons to conduct electricity (by moving around the structure). However, they have free moving ions or electrons in a molten or aqueous state.
These ions allow ionic bonds to conduct electricity by freely moving around the structure.
- They are soluble in water and insoluble in organic compounds (Higher):
Most ionic compounds are soluble in water because water molecules strongly attract the charged ions. Therefore, they dissociate in water and become solvated.
This is the reason why ionic compounds are soluble in water.
On the other hands, they are insoluble in organic solvents because the organic solvents are non-polar. In simple words, they cannot break or dissolve the ionic compounds.
Conclusion:
Thank you very much for reading this article about ionic bonds. If you have any questions, do leave them below. I will try my best to answer them.
To recap, we discussed ions (cations and anions), the formation of ionic bonds, how to find out the formula of ionic bonds, and their properties.
This information may be useful for IGCSE Chemistry and GCE O Level Chemistry students. Stay tuned for further useful resources.

Comments
Post a Comment